While soil acts as a buffer, forgiving minor errors in feeding, hydroponics offers no such safety net. It offers something better: total control. By mastering the chemistry of your reservoir, you can achieve growth rates significantly faster than traditional gardening.
However, this control requires precision. As discussed in Hydroponics 101: The Ultimate Beginner's Guide, the water in your system is the lifeblood of your garden. In this technical guide, we will move beyond the basics and dive deep into the specific chemical requirements needed to sustain vigorous plant life.
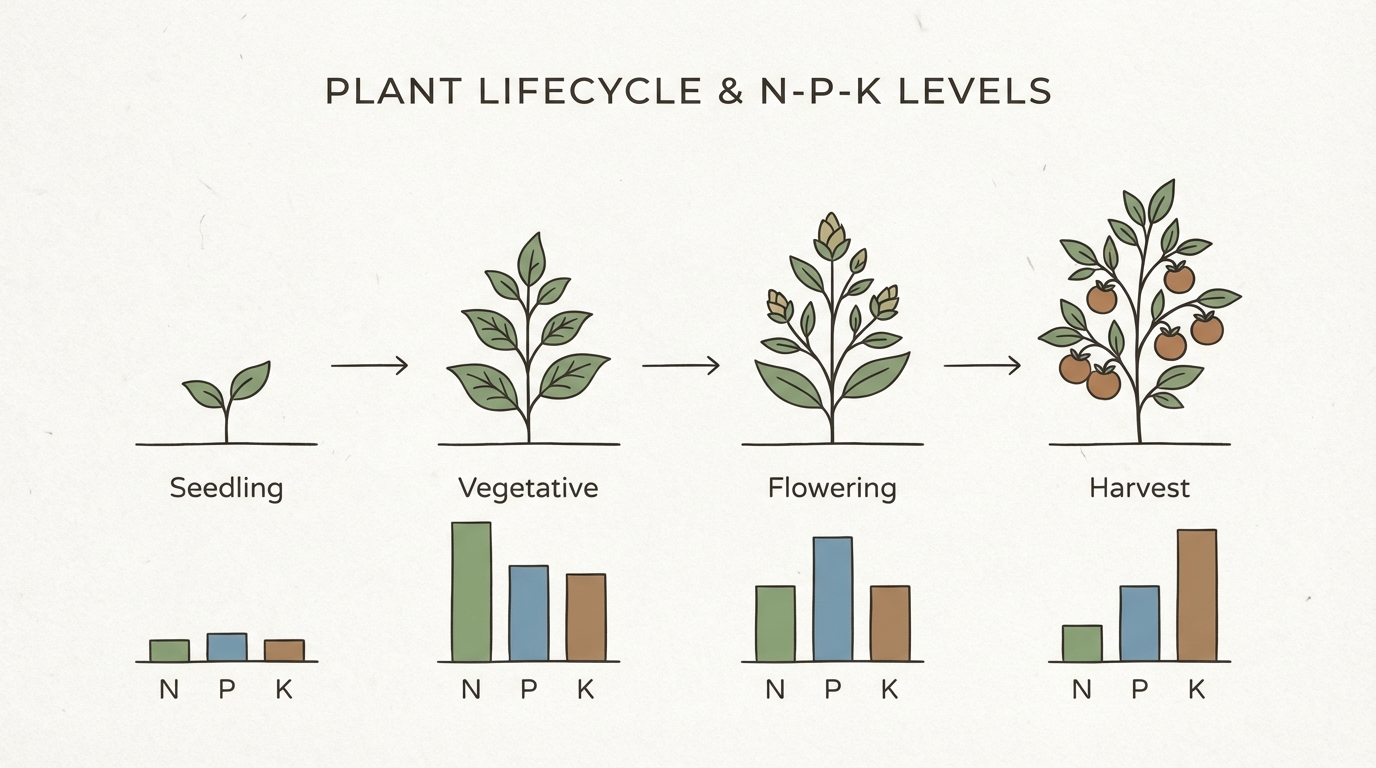
Decoding the N-P-K Ratio
Every bottle of fertilizer, whether organic or synthetic, displays three numbers: N-P-K. These stand for Nitrogen (N), Phosphorus (P), and Potassium (K), the primary macronutrients required for plant survival.
- Nitrogen (N): The engine of vegetative growth. It drives leaf and stem development. High nitrogen is crucial during the early stages of leafy greens and herbs.
- Phosphorus (P): Essential for photosynthesis and energy transfer. It is critical during the flowering and rooting stages.
- Potassium (K): Regulates water uptake and enzyme activation. It improves the overall hardiness of the plant and the quality of fruit.
While specific crops listed in our guide to the Best Plants to Grow in Hydroponics have unique preferences, the general rule of thumb involves shifting ratios based on the growth phase. You generally want a higher N ratio during the vegetative stage and a higher P-K ratio when the plant begins to fruit or flower.
The Gatekeeper: Understanding pH
You can have the most expensive, high-quality nutrients in your reservoir, but if your pH is off, your plants will starve. This phenomenon is known as Nutrient Lockout.
pH is measured on a scale of 0 to 14, with 7 being neutral. Hydroponic plants generally prefer a slightly acidic environment, typically between 5.5 and 6.5.
Why the Range Matters
At the chemical level, nutrients exist as ions. The pH of the solution affects the solubility of these ions. If the pH rises too high (alkaline), essential micronutrients like Iron and Manganese precipitate out of the solution and become unavailable to the roots. If it drops too low (acidic), macronutrients like Calcium and Magnesium are locked out.
Maintaining this range is a daily task. In active systems, such as those described in 6 Types of Hydroponic Systems Explained, plants excrete waste products through their roots that naturally alter the pH of the reservoir. Regular testing is mandatory.
Measuring Strength: EC and PPM
Two metrics help us understand how much food is in the water:
- EC (Electrical Conductivity): Pure water does not conduct electricity well. Dissolved salts (nutrients) conduct electricity. The higher the EC, the more nutrients are in the water.
- PPM (Parts Per Million): A conversion of EC into a concentration metric. It tells you the density of dissolved solids.
Young plants (seedlings) require a low EC (0.8 - 1.2), while heavy feeders like tomatoes may require an EC as high as 2.0 - 2.4. Over-fertilizing leads to "nutrient burn," characterized by crispy, brown leaf tips.

The Mixing Ritual: A Step-by-Step Guide
Creating a nutrient solution is a chemical process. The order in which you mix ingredients prevents them from reacting with each other before they reach the plant.
The Golden Protocol
- Start with Fresh Water: Fill your reservoir. If using tap water, let it sit for 24 hours to allow chlorine to dissipate, or use an RO (Reverse Osmosis) filter.
- Add Micro-Nutrients First: If using a 3-part nutrient series, always add the "Micro" bottle first to the water. Stir well. Calcium in the Micro can bond with sulfur or phosphorus in other bottles if mixed directly in concentrated form, creating distinct white flakes (precipitate) that plants cannot eat.
- Add Grow/Bloom Nutrients: Add the remaining macro-nutrients one by one, stirring thoroughly between each addition.
- Check and Adjust pH Last: Nutrients are acidic. Adding them will naturally lower your water's pH. Only after all nutrients are mixed should you measure the pH. Use "pH Up" (Base) or "pH Down" (Acid) solutions to land in the 5.5 - 6.5 sweet spot.
Troubleshooting Common Issues
Even with the Ultimate Guide to hydroponic gardening at your disposal, issues will arise. Here is how to diagnose water chemistry problems:
- Yellowing Leaves (Chlorosis): often indicates Nitrogen deficiency or pH lockout preventing Nitrogen uptake. Check pH first before adding more food.
- Purple Stems: Can indicate a Phosphorus deficiency or temperature stress.
- Cloudy Water / Bad Smell: Root rot. This is often caused by water temperatures above 72°F (22°C) or lack of oxygenation.

Equipment: Cost vs. Accuracy
Maintaining this balance requires tools. You can use liquid drop test kits (cheap but subjective) or digital meters (expensive but precise). While digital meters require calibration, they offer the accuracy needed for serious growers. For a deeper dive into budgeting for this gear, refer to our analysis on DIY Hydroponic Setup vs. Kits: Cost Analysis.
Conclusion
Managing nutrients and pH is not a "set it and forget it" task. It is a daily ritual of observation and adjustment. By keeping your reservoir within the optimal chemical parameters, you ensure that your plants spend their energy on growing fruit and foliage rather than fighting for survival.
